Avicanna Reports Full Year 2024 Audited Financial Statement
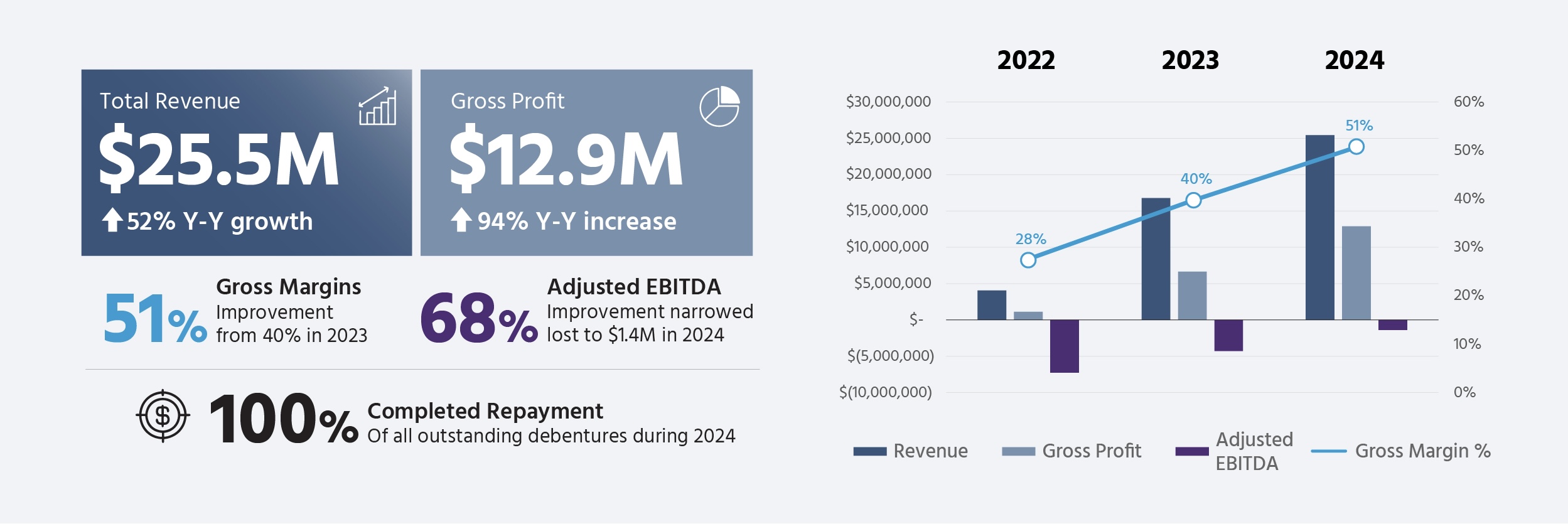
2024 Revenue of $25.5M, a 52% increase from 2023
Consolidated Gross Margins of 51%, Gross Profits of $12.9M, a 94% increase from 2023
TORONTO, April 14, 2025 (GLOBE NEWSWIRE) -- Avicanna Inc. (“Avicanna” or the “Company) (TSX: AVCN) (OTCQX: AVCNF) (FSE: 0NN), a biopharmaceutical company focused on the development, manufacturing, and commercialization of plant-derived cannabinoid-based products, is pleased to announce that its full year 2024 results, audited financial statements with management’s discussion and analysis have been filed.
Management Commentary:
"We are proud to report our most successful year to date, marked by improved financial results and continued advancements in our commercial, R&D and clinical programs. In 2024, we strengthened our financial foundation, achieved self-sufficiency, and established a solid basis for further growth, international expansion, and innovation. We remain committed to our mission of advancing cannabinoid-based medicine and are energized by the prospects that lie ahead as we continue to expand and strengthen our core business pillars " stated Aras Azadian, CEO of Avicanna.
Full Year 2024 Financial Highlights:
- Annual Revenue: Achieved record revenue of $25.5 million for the year ended December 31, 2024, supported by $6.6 million in revenue for the fourth quarter. This represents a 52% year-over-year growth from 2023 and was driven by both Canadian and international business segments.
- Gross Profit Growth: Reported a year-over-year gross profit of $12.9 million, representing an increase of 94% compared to 2023.
- Gross Margin Improvement: Consolidated Gross margins improved to 51% in 2024, representing an increase of 40% in 2023. This improvement in gross margin is attributed to continued optimization efforts and an increased licensing and service revenue.
- Adjusted EBITDA: Annual adjusted EBITDA improved by 68% from 2023, narrowing the loss to $1.4 million for 2024, compared to a loss of $4.3 million in 2023.
- Debt Repayment: The Company repaid the outstanding principal balance of $1.3 million on its Non-Convertible Debentures issued in August 2023.
Other 2024 Highlights:
Initiation of Medical Cannabis Real World Evidence Study by MyMedi.ca (“RWE Study”): A prospective, non-interventional, observational study aimed to enroll 1,000 patients across Canada to better understand the potential therapeutic use of medical cannabis and potential impact of medical cannabis on pain, sleep, anxiety, depression, and epilepsy. The RWE Study is led by Dr. Hance Clarke, President of The Canadian Pain Society and the CCIC is leading the RWE Study.
Canadian Commercial Advancements in 2024: The Company completed the year with 42 proprietary commercial SKUs and 136 unique commercial listings. The Company sold approximately 200,000 units during 2024, an increase of 8% from 2023.
Completion of Study in Patients with Epidermolysis Bullosa at The Hospital for Sick Children Evaluating Wound Healing, Pain, and Itch (“EB Study”): The EB Study led by Elena Pope, MD, M.Sc., FRCPC, Head of Dermatology at The Hospital for Sick Children in Toronto, evaluated the tolerability and efficacy of a proprietary 3% CBD topical cream in patients with epidermolysis bullosa. 55% of patients enrolled in the Study reported improvements in wound healing, 45% displayed wound stability.
Completion of Topical Gel Observational Real-World Evidence Study in Patients with Musculoskeletal Pain and Inflammation (“MPI RWE Study”): The MPI RWE Study evaluated patient-reported efficacy of the proprietary Transdermal Gel containing 2% CBD and 1% CBG on a range of clinical conditions including arthritis, osteoarthritis, rheumatoid arthritis, fibromyalgia, muscle and joint pain, localized pain, and post-surgical pain. The MPI RWE Study reported a meaningful improvement in overall Musculoskeletal Health Questionnaire scores (p<0.001) as compared from baseline to one month, specifically, there was a 35.4% improvement reported in health-related domains including symptoms, physical functioning, daily activities and work.
Avicanna LATAM SAS obtained indication-specific Drug Registration in Colombia with Trunerox™: Trunerox™ was approved in Colombia by the Colombian National Institute of Drug and Food Surveillance (El Instituto Nacional de Vigilancia de Medicamentos y Alimentos – “INVIMA”) as a drug for the treatment of severe seizures related to Lennox-Gastaut Syndrome and Dravet Syndrome. The approval permits Avicanna LATAM SAS to manufacture and commercialize Trunerox™ in Colombia for the approved indications which are two rare epileptic disorders classified as epileptic encephalopathies. Trunerox™ has not been approved as a drug in Canada by Health Canada.
Avicanna Completed the First Delivery of Proprietary Topical Products to a Multinational Pharmaceutical Company in 2024: The products include 3% CBD localized cream and the 2% CBD and 0.5% CBG transdermal gel utilizing the Company’s patented deep tissue technology. The 3% CBD localized cream, as well as the 2% CBD and 0.5% CBG transdermal gel products completed human irritation and real-world evidence studies.
United States Patent and Trademark Office (“USPTO”) Issued Patent No. US 12,064,461 B2 and No. US 11,998,632 B2 to Avicanna: US Patent No. US 12,064,461 B2 covers Avicanna’s deep penetrating topical cannabinoid composition and methods for treating musculoskeletal inflammation and pain; and US Patent No. US 11,998,632 B2 covers Avicanna’s “SEDDS” or the self-emulsifying drug delivery system technology for oral cannabinoid composition and methods of treating neuropathic pain.
2024 Earnings Call and Corporate Update:
The Company’s CEO, Mr. Aras Azadian, and CFO, Mr. Phillip Cardella, will host an earnings call and corporate update at 8:30 am ET, Tuesday, April 22, 2025. Interested parties may register by clicking below:
About Avicanna:
Avicanna is a commercial-stage international biopharmaceutical company focused on the advancement and commercialization of cannabinoid-based products and formulations for the global medical and pharmaceutical market segments. Avicanna has an established scientific platform including R&D and clinical development leading to the commercialization of more than thirty proprietary, evidence-based finished products and supporting four commercial stage business pillars.
- Medical Cannabis formulary (RHO Phyto™): The formulary offers a diverse range of proprietary products including oral, sublingual, topical, and transdermal deliveries with varying ratios of cannabinoids, supported by ongoing patient and medical community education. RHO Phyto is an established brand in Canada currently available nationwide across several channels and expanding into new international markets.
- Medical cannabis care platform (MyMedi.ca): MyMedi.ca is a medical cannabis care platform formed with the aim to better serve medical cannabis patients’ needs and enhance the medical cannabis patients’ journey. MyMedi.ca is operated by Northern Green Canada Inc. and features a diverse portfolio of products and bilingual pharmacist-led patient support programs. MyMedi.ca also provides specialty services to distinct patient groups such as veterans and collaborates with public and private payers for adjudication and reimbursement. MyMedi.ca provides educational resources to the medical community to facilitate the incorporation of medical cannabis into health care regimens.
- Pharmaceutical pipeline: Leveraging Avicanna’s scientific platform, vertical integration, and real-world evidence, Avicanna has developed a pipeline of proprietary, indication-specific cannabinoid-based candidates that are in various stages of clinical development. These cannabinoid-based candidates aim to address unmet needs in the areas of dermatology, chronic pain, and various neurological disorders.
- Active pharmaceutical ingredients (Aureus Santa Marta™): Active pharmaceutical ingredients supplied by the Company’s majority owned subsidiary Santa Marta Golden Hemp SAS (“SMGH”) is a commercial-stage business dedicated to providing various forms of high-quality CBD, THC and CBG to the Company’s international partners for use in the development and production of food, cosmetics, medical, and pharmaceutical products. SMGH also forms part of the Company’s supply chain and is a source of reliable input products for its consumer retail, medical cannabis, and pharmaceutical products globally.
SOURCE Avicanna Inc.
Stay Connected
For more information about Avicanna, visit our website or contact Ivana Maric by email at info@avicanna.com.
Cautionary Note Regarding Forward-Looking Information and Statements
This news release contains “forward-looking information” within the meaning of applicable securities laws. Forward-looking information contained in this news release may be identified by the use of words such as, “may”, “would”, “could”, “will”, “likely”, “expect”, “anticipate”, “believe”, “intend”, “plan”, “forecast”, “project”, “estimate”, “outlook” and other similar expressions. Forward-looking information contained in this news release includes, without limitation, statements related to the Offering, the use of proceeds of the Offering, the receipt of all approvals of the Toronto Stock Exchange in connection with the Offering, statements with respect to the Company’s future business operations, the opinions or beliefs of management and future business goals. Although the Company believes that the expectations and assumptions on which such forward looking information is based are reasonable, undue reliance should not be placed on the forward-looking information because the Company can give no assurance that they will prove to be correct. Actual results and developments may differ materially from those contemplated by these statements. Forward-looking information is subject to a variety of risks and uncertainties that could cause actual events or results to differ materially from those projected in the forward-looking information. Such risks and uncertainties include, but are not limited to current and future market conditions, including the market price of the common shares of the Company, and the risk factors set out in the Company’s annual information form dated April 11, 2025, filed with the Canadian securities regulators and available under the Company’s profile on SEDAR+ at www.sedarplus.ca. The statements in this news release are made as of the date of this release. The Company disclaims any intent or obligation to update any forward-looking information, whether as a result of new information, future events or results or otherwise, other than as required by applicable securities laws.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/796929bf-c32f-430f-bebb-74eb10be0a8f

